Electronic Configurations in Periods :
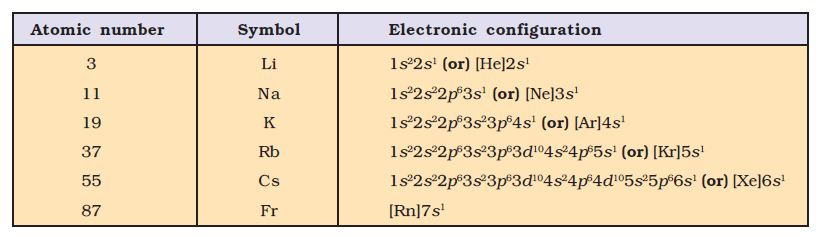
`=>` The period indicates the value of `n` for the outermost or valence shell.
● In other words, successive period in the Periodic Table is associated with the filling of the next higher principal energy level (`n = 1`, `n = 2`, etc.).
● It can be readily seen that the number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.
`=>` The first period (`n = 1`) starts with the filling of the lowest level (`1s`) and therefore has two elements — hydrogen `(1s^1)` and helium `(1s^2)` when the first shell `(K)` is completed.
`=>` The second period `(n = 2)` starts with lithium and the third electron enters the `2s` orbital.
● The next element, beryllium has four electrons and has the electronic configuration `1s^2 , 2s^2`.
● Starting from the next element boron, the `2p` orbitals are filled with electrons when the `L` shell is completed at neon `(2s^2 , 2p^6)`.
● Thus there are `8` elements in the second period.
`=>` The third period `(n = 3)` begins at sodium, and the added electron enters a `3s` orbital.
● Successive filling of `3s` and `3p` orbitals gives rise to the third period of `8` elements from sodium to argon.
`=>` The fourth period `(n = 4)` starts at potassium, and the added electrons fill up the `4s` orbital.
● Here before the `4p` orbital is filled, filling up of `3d` orbitals becomes energetically favourable and we come across the so called `3d` `text(transition series)` of elements.
● This starts from scandium `(Z = 21)` which has the electronic configuration `3d^1 , 4s^2`.
● The `3d` orbitals are filled at zinc `(Z=30)` with electronic configuration `3d^(10) , 4s^2`.
● The fourth period ends at krypton with the filling up of the `4p` orbitals.
● Altogether we have `18` elements in this fourth period.
`=>` The fifth period `(n = 5)` beginning with rubidium is similar to the fourth period and contains the `4d` transition series starting at yttrium `(Z = 39)`.
● This period ends at xenon with the filling up of the `5p` orbitals.
● The sixth period `(n = 6)` contains `32` elements and successive electrons enter `6s, 4f, 5d` and `6p` orbitals, in the order — filling up of the `4f` orbitals begins with cerium `(Z = 58)` and ends at lutetium `(Z = 71)` to give the `4f`-inner transition series which is called the `text(lanthanoid series)`.
`=>` The seventh period `(n = 7)` is similar to the sixth period with the successive filling up of the `7s, 5f, 6d` and `7p` orbitals and includes most of the man-made radioactive elements.
● This period will end at the element with atomic number `118` which would belong to the noble gas family.
● Filling up of the `5f` orbitals after actinium `(Z = 89)` gives the `5f`-inner transition series known as the `text(actinoid series)`.
`=>` The `4f` and `5f`-inner transition series of elements are placed separately in the Periodic Table to maintain its structure and to preserve the principle of classification by keeping elements with similar properties in a single column.
● In other words, successive period in the Periodic Table is associated with the filling of the next higher principal energy level (`n = 1`, `n = 2`, etc.).
● It can be readily seen that the number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.
`=>` The first period (`n = 1`) starts with the filling of the lowest level (`1s`) and therefore has two elements — hydrogen `(1s^1)` and helium `(1s^2)` when the first shell `(K)` is completed.
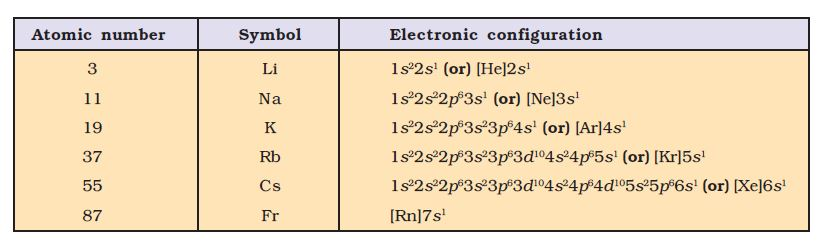
`=>` The second period `(n = 2)` starts with lithium and the third electron enters the `2s` orbital.
● The next element, beryllium has four electrons and has the electronic configuration `1s^2 , 2s^2`.
● Starting from the next element boron, the `2p` orbitals are filled with electrons when the `L` shell is completed at neon `(2s^2 , 2p^6)`.
● Thus there are `8` elements in the second period.
`=>` The third period `(n = 3)` begins at sodium, and the added electron enters a `3s` orbital.
● Successive filling of `3s` and `3p` orbitals gives rise to the third period of `8` elements from sodium to argon.
`=>` The fourth period `(n = 4)` starts at potassium, and the added electrons fill up the `4s` orbital.
● Here before the `4p` orbital is filled, filling up of `3d` orbitals becomes energetically favourable and we come across the so called `3d` `text(transition series)` of elements.
● This starts from scandium `(Z = 21)` which has the electronic configuration `3d^1 , 4s^2`.
● The `3d` orbitals are filled at zinc `(Z=30)` with electronic configuration `3d^(10) , 4s^2`.
● The fourth period ends at krypton with the filling up of the `4p` orbitals.
● Altogether we have `18` elements in this fourth period.
`=>` The fifth period `(n = 5)` beginning with rubidium is similar to the fourth period and contains the `4d` transition series starting at yttrium `(Z = 39)`.
● This period ends at xenon with the filling up of the `5p` orbitals.
● The sixth period `(n = 6)` contains `32` elements and successive electrons enter `6s, 4f, 5d` and `6p` orbitals, in the order — filling up of the `4f` orbitals begins with cerium `(Z = 58)` and ends at lutetium `(Z = 71)` to give the `4f`-inner transition series which is called the `text(lanthanoid series)`.
`=>` The seventh period `(n = 7)` is similar to the sixth period with the successive filling up of the `7s, 5f, 6d` and `7p` orbitals and includes most of the man-made radioactive elements.
● This period will end at the element with atomic number `118` which would belong to the noble gas family.
● Filling up of the `5f` orbitals after actinium `(Z = 89)` gives the `5f`-inner transition series known as the `text(actinoid series)`.
`=>` The `4f` and `5f`-inner transition series of elements are placed separately in the Periodic Table to maintain its structure and to preserve the principle of classification by keeping elements with similar properties in a single column.